IRO Business Providing Total Business Solutions Starts on Full Scale
-Elimination of drug lag and drug loss, and support for foreign biotech companies entering the Japanese market-
-
2024.04.23 News
2024/4/23, EPS Holdings, Inc., News Release --- EPS Holdings, Inc. (Head Office: Shinjuku-ku, Tokyo, Japan; Representative Director: Hao Yan; hereafter, “EPSHD”) announces that it has started IRO (Innovative Research Organization) business operations on a full scale, to provide total solutions to foreign biotech companies and domestic healthcare venture companies.
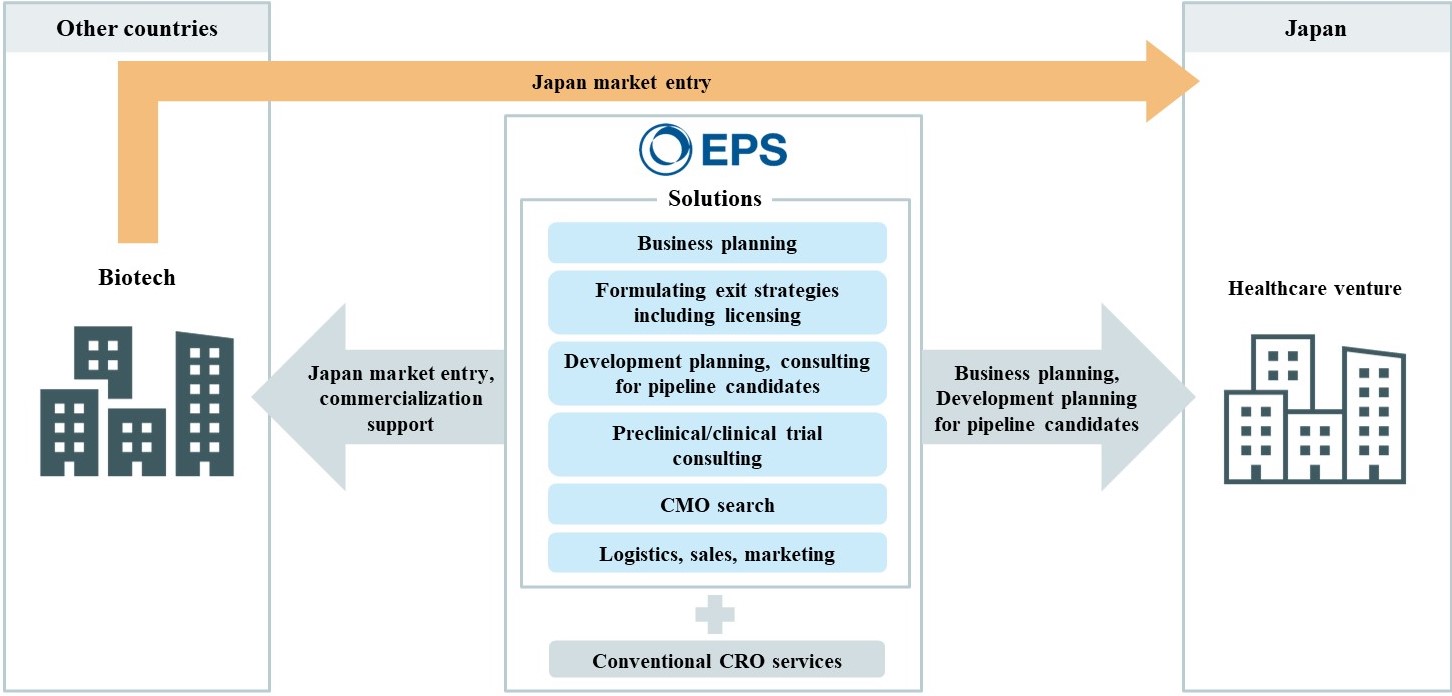
1. Description of IRO Business
In our IRO business, we will provide greatly needed CRO services to the clients, who are foreign biotech companies and domestic healthcare ventures, in a form of total solutions that go beyond those conventionally provided by CROs. This includes support of business planning (support for entering the Japanese market in case of foreign biotech companies), support of exit strategy formulation (including licensing-out to partner companies), consulting on development planning for individual candidates in pipelines, preclinical and clinical trial consulting, CMO search support and support of logistics, sales, and marketing.
EPSHD has provided total IRO business solutions to domestic healthcare venture companies since the establishment of this business in 2022, and it will now be developed on a full scale to include foreign biotech companies as well.
In our IRO business, we will provide greatly needed CRO services to the clients, who are foreign biotech companies and domestic healthcare ventures, in a form of total solutions that go beyond those conventionally provided by CROs. This includes support of business planning (support for entering the Japanese market in case of foreign biotech companies), support of exit strategy formulation (including licensing-out to partner companies), consulting on development planning for individual candidates in pipelines, preclinical and clinical trial consulting, CMO search support and support of logistics, sales, and marketing.
EPSHD has provided total IRO business solutions to domestic healthcare venture companies since the establishment of this business in 2022, and it will now be developed on a full scale to include foreign biotech companies as well.
2.Background and Overview of Business
At present, drug lag and drug loss are serious issues in Japan. Some of the reasons for this are the complexity of Japan’s clinical trial procedures and pharmaceutical regulations, and the framework for implementing clinical trials peculiar to Japan, which includes academia. This presents high hurdles to market entry for foreign biotech companies.
Expansion of our IRO business to include foreign biotech companies and the start of its full-scale operation will contribute to eliminate drug loss by solving the above issues faced by foreign biotech companies and supporting their entry into the Japanese market.
Recently, the EPSHD group company EPS Medical Consultancy (Japan) Co., Ltd. (Head Office: Tokyo, Japan; Representative Director: Kosuke Kuronuma) concluded consulting agreements with several Chinese emerging biotech companies on business development and clinical development in Japan, as Japan’s first IRO business. EPS has now started providing IRO total business solutions in collaboration with other EPS group companies.
At present, drug lag and drug loss are serious issues in Japan. Some of the reasons for this are the complexity of Japan’s clinical trial procedures and pharmaceutical regulations, and the framework for implementing clinical trials peculiar to Japan, which includes academia. This presents high hurdles to market entry for foreign biotech companies.
Expansion of our IRO business to include foreign biotech companies and the start of its full-scale operation will contribute to eliminate drug loss by solving the above issues faced by foreign biotech companies and supporting their entry into the Japanese market.
Recently, the EPSHD group company EPS Medical Consultancy (Japan) Co., Ltd. (Head Office: Tokyo, Japan; Representative Director: Kosuke Kuronuma) concluded consulting agreements with several Chinese emerging biotech companies on business development and clinical development in Japan, as Japan’s first IRO business. EPS has now started providing IRO total business solutions in collaboration with other EPS group companies.
3.Future Outlook
To support the entry of foreign biotech companies into the Japanese market and the business operation of domestic healthcare venture companies, EPSHD aims to greatly expand the IRO business in times to come. In the medium to long-term, the size of this business is expected to be from several billion yen to 10 billion yen.
By actively accelerating the overseas expansion of our IRO business, we will contribute to the entry of foreign biotech companies into the Japanese market and elimination of drug lag and drug loss in Japan.
To support the entry of foreign biotech companies into the Japanese market and the business operation of domestic healthcare venture companies, EPSHD aims to greatly expand the IRO business in times to come. In the medium to long-term, the size of this business is expected to be from several billion yen to 10 billion yen.
By actively accelerating the overseas expansion of our IRO business, we will contribute to the entry of foreign biotech companies into the Japanese market and elimination of drug lag and drug loss in Japan.
[EPS Holdings, Inc.]
Since its establishment as a CRO pioneer in 1991, EPSHD has provided solutions regarding drug development, post-marketing development, marketing and sales, as well as consultation. In addition, it has been a healthcare solution provider offering new value to pharmaceutical companies, medical device companies, hospitals, clinics and academia through big data & AI, and regenerative medicine initiatives. In 2021, we established a drug discovery business to support the clinical development of drug candidates originating in academia as well as in domestic and foreign bio-ventures, and started a business providing marketing support in Japan and other countries.
[EPS Medical Consultancy (Japan) Co., Ltd.]
The role of EPS Medical Consultancy (Japan) Co., Ltd. is as a business support company whose major business involves linking up pharmaceutical and medical device development in Japan, China and other parts of Asia. In its business to support drug discovery, it provides one-stop solutions to Japanese healthcare ventures as well as emerging biotech companies in China and other parts of Asia. The following gives details of support provided:
1.Support of Japanese healthcare ventures and support of emerging biotech companies in China and the rest of Asia planning business expansion into the Japanese market (including In/Out licensing support as exit strategy), consulting regarding development and planning for individual candidates in pipelines and 2.Clinical trial-related services to support advances of Japanese pharmaceutical companies into the Chinese market.
Since its establishment as a CRO pioneer in 1991, EPSHD has provided solutions regarding drug development, post-marketing development, marketing and sales, as well as consultation. In addition, it has been a healthcare solution provider offering new value to pharmaceutical companies, medical device companies, hospitals, clinics and academia through big data & AI, and regenerative medicine initiatives. In 2021, we established a drug discovery business to support the clinical development of drug candidates originating in academia as well as in domestic and foreign bio-ventures, and started a business providing marketing support in Japan and other countries.
[EPS Medical Consultancy (Japan) Co., Ltd.]
The role of EPS Medical Consultancy (Japan) Co., Ltd. is as a business support company whose major business involves linking up pharmaceutical and medical device development in Japan, China and other parts of Asia. In its business to support drug discovery, it provides one-stop solutions to Japanese healthcare ventures as well as emerging biotech companies in China and other parts of Asia. The following gives details of support provided:
1.Support of Japanese healthcare ventures and support of emerging biotech companies in China and the rest of Asia planning business expansion into the Japanese market (including In/Out licensing support as exit strategy), consulting regarding development and planning for individual candidates in pipelines and 2.Clinical trial-related services to support advances of Japanese pharmaceutical companies into the Chinese market.
Contact: EPS Holdings, Inc.
Corporate Communication Office
E-mail:pr@eps.co.jp